Crystallization behavior of citric acid based on solution speciation and growth kinetics†
Abstract
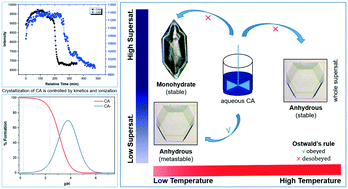
Ostwald's rule of stages is one of the most important empirical rules to understand the crystallization behavior of crystalline materials, however, it may not be always satisfied. Herein, we demonstrate that crystallization outcomes of citric acid (CA) in aqueous solutions violate this rule. The anhydrous phase was found to nucleate at the whole temperature range (below or above the transition point of the system), whereas, the monohydrate form only nucleated at high supersaturation below the transition point. The unexpected appearance of the anhydrous phase is shown to have been influenced by solution ionization and its crystallization kinetic advantage over the monohydrate form.


 Please wait while we load your content...
Please wait while we load your content...