Prediction of stable energetic beryllium pentazolate salt under ambient conditions†
Abstract
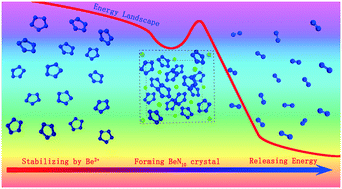
BeN10 has the highest nitrogen mass concentration (up to 94%) around metal pentazolates. However, it is still a challenge to directly confirm the microstructure and property of the most stable BeN10 under ambient conditions due to the metastable feature of energetic materials. In the present study, we directly detected a new BeN10 crystalline structure under atmospheric pressure by a constrained crystal structure search method. In comparison with the phase searched from high pressures, this new BeN10 shows much lower formation energy (1.37 eV per formula). Its dynamic and thermodynamic stabilities were further evaluated via phonon spectrum simulations and molecular dynamics simulations, and the calculated results show that this new BeN10 salt has good stability up to 600 K. The bond analysis indicates that the bond strength of Be–N is a key factor to stabilize BeN10. Moreover, the simulations of electronic structures and the absorption spectrum indicate that this new BeN10 salt is an intrinsic insulator along with good optical stability. The oxidative decomposition of the BeN10 salt could release energy up to 5.36 kJ g−1, which indicates that BeN10 is a kind of potential excellent energetic material.


 Please wait while we load your content...
Please wait while we load your content...