The hydrothermal synthesis of ZnSn(OH)6 and Zn2SnO4 and their photocatalytic performances
Abstract
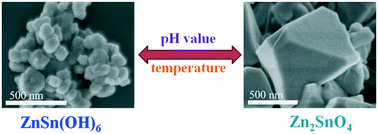
ZnSn(OH)6 and Zn2SnO4 were synthesized by a simple hydrothermal method. The influences of reaction conditions on the formation of ZnSn(OH)6 and Zn2SnO4 were investigated in detail. ZnSn(OH)6 and Zn2SnO4 showed highly efficient photocatalytic activity for the degradation of methylene blue under ultraviolet light irradiation. Kinetic studies using radical scavenger technologies suggested that hydroxyl radicals were the dominant photooxidants. On the basis of the experimental results, the excellent photocatalytic activities of ZnSn(OH)6 were mainly attributed to the hydroxyl groups which were part of the ZnSn(OH)6 lattice. The recovery of hydroxyl groups could make up for the consumption.


 Please wait while we load your content...
Please wait while we load your content...