Design, synthesis and investigations of a series of energetic salts through the variation of amines and concentration of picrate anions†
Abstract
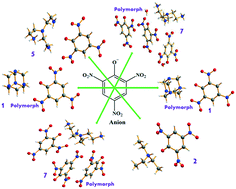
Eight new energetic salts combining N-bases (amines) with picrate ions were synthesized in high yields and characterized by NMR (1H and 13C), IR spectroscopy, elemental analysis, and differential thermal analysis. Single-crystal structural studies were performed to confirm the structures, packing, and various interactions in the salts. Interestingly, salts 1 and 7 exist in different polymorphic forms and the effect of solvent on polymorphism was examined (i.e. solvatomorphism). Thermal stability and impact and friction sensitivities were also determined to understand the relationship between their structures and properties. Salt 3 with hydrazinium cations showed higher performance (d = 1.82 g cm−3; Td = 255.2 °C; D = 7.95 km s−1; P = 28.2 GPa; IS ≥ 30 J; FS ≥ 350 N) towards energetic applications. However, salts 4, 7, and 8 also exhibit good performance parameters, which are marginally better as compared to TNT. The tuning of the physiochemical properties and performances was carried out with the modification of the cationic-amine molecules and the molar ratio of the reactants.


 Please wait while we load your content...
Please wait while we load your content...