Effect of electron-withdrawing moieties on mechanochromism of phenothiazine derivatives†
Abstract
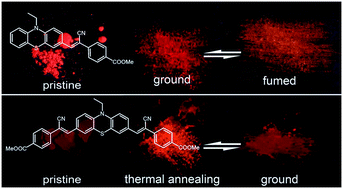
Two phenothiazine derivatives with two (CPBM) or four (CPDBM) electron-withdrawing moieties were synthesized, and their responsive properties to mechanical force were investigated and compared. CPDBM had absorption and emission spectra with longer wavelengths than CPBM in hexane. Quantum chemical calculation and electrochemical measurement were conducted. The longer CPDBM wavelengths could be ascribed to the decrease in the LUMO energy level owing to the existence of additional electron-withdrawing groups. In the crystal state, CPBM aggregated into dimers with a short distance and exhibited reversible mechanofluorochromism (MFC). The fluorescence color exhibited reversible conversion between orange and red under alternative force and fuming stimuli. Long and thin dark red crystals of CPDBM with weak red fluorescence were obtained, and the fluorescence did not change after grinding. Thermal annealing of the dark red crystals produced red solids with orange fluorescence and strong red emission after grinding. Fuming and thermal annealing led to orange fluorescence, indicating MFC behavior.


 Please wait while we load your content...
Please wait while we load your content...