Synthesis and solid-state characterization of diclofenac imidazolium monohydrate: an imidazolium pharmaceutical ionic liquid†
Abstract
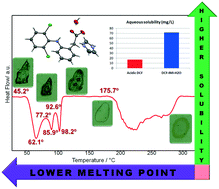
A new imidazolium hydrated salt (DCF–IMI–H2O) of the nonsteroidal anti-inflammatory drug diclofenac (DCF) was synthesized by solvent evaporation. This salt was fully characterized by X-ray diffraction (SCXRD and PXRD), infrared spectroscopy (FT-IR) and thermal analysis (TGA, DSC, and HSM) techniques. The salt formation is driven by deprotonation of the drug's carboxylic group, with proton transfer to the nitrogen atom of the imidazole ring. The water molecule plays an important role in the 3D arrangement observed in the crystal lattice, acting as a bridge between adjacent DCF and coformer molecules. DCF–IMI–H2O exhibits an improved solubility profile when compared with the pure form of DCF, and similar values when compared with sodium and potassium salts. This new salt proved stable under 75% relative humidity for one week and is stable after six-months of storage under ambient conditions. The study of its thermal stability showed that a dehydration process starts at around 45 °C, leading to the probable formation of an unstable anhydrous form, which melts around 92 °C. Spectroscopy studies showed that most of the salt is ionized when in an amorphous state (liquid). These behaviors feature the new compound as an ionic liquid (IL), i.e., organic salt with a melting point below 100 °C. Pharmaceutical ILs have been increasingly exploited in the past few years due to their improved properties regarding drug solubility, formulation and delivery (topical, transdermal and oral). Thus, the salt depicted herein becomes a potent candidate for development of novel/improved DCF delivery systems.


 Please wait while we load your content...
Please wait while we load your content...