Dimensional evolution in hydrated K+-bearing uranyl sulfates: from 2D-sheets to 3D-frameworks†
Abstract
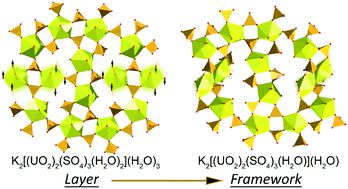
In the course of the investigation of the phase formation in a K-bearing uranyl sulfate system under low-temperature hydrothermal conditions, stepwise crystallization of eight chemically and structurally distinct uranyl-bearing phases, including six novel compounds, has been observed. The crystal structures of four new compounds are based on units with novel topologies, one of which has never been observed in uranyl sulfates and three others are unprecedented for the structural chemistry of inorganic oxysalts. The obtained phases were analyzed using single-crystal XRD, EDX, topological analysis and information-based structural complexity calculations. The analysis of the chemical evolution in the system shows that the amount of U and S in the crystalline phases increases and decreases, respectively, driven by the increase of the pH value. The solution was heated to 55 °C, which appears to be insufficient for the formation of zippeite-type K-bearing uranyl sulfates. The analysis of the behavior of structural complexity indicates that most complex compounds crystallize on the borders between stability fields of stable compounds with less complex structural architectures.


 Please wait while we load your content...
Please wait while we load your content...