On the impact of metal ion proportion on the physical properties of heterometallic metal–organic frameworks†
Abstract
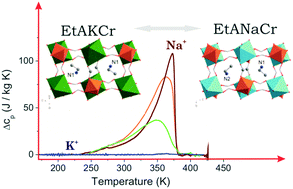
Indications of desirable polar order in ethylammonium C2H5NH3(EtA) metal formate [EtA][Na0.5Cr0.5(HCOO)3] and the lack of it in [EtA][K0.5Cr0.5(HCOO)3] raised important questions on the origin of structural phase transition and dipolar dynamics in these kinds of compounds. In this paper we provide results of experimental studies performed on the mixed frameworks, which may give more insight into understanding these phenomena. We report the synthesis of four heterometallic metal–organic frameworks [CH3CH2NH3][KxNa0.5−xCr0.5(HCOO)3] with various compositions (x = 0, 0.04, 0.22, and 0.5). Broadband dielectric spectroscopy assisted by vibrational spectroscopy allows us to determine the role of EtA+ motions in the structural phase transition in the investigated compounds. We clearly demonstrate that the increase of the K/Na ratio results in freer translational movements of the EtA+ cations.


 Please wait while we load your content...
Please wait while we load your content...