Tetrachloroplatinate(ii) anion as a square-planar tecton for crystal engineering involving halogen bonding†
Abstract
In the X-ray structures of the 1 : 1 adduct of [Ph3P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N
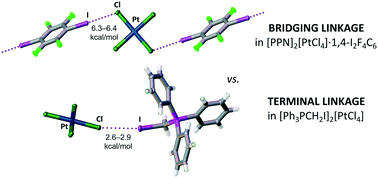
N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PPh3]2[PtCl4] with 1,4-diiodotetrafluorobenzene (1·FIB) and the salt [Ph3PCH2I]2[PtCl4] (2), the tetrachloroplatinate(II) anion behaves as a halogen bond (XB)-accepting tecton toward σ-hole-donating organohalogen species to form either C–I⋯Cl–PtCl2–Cl⋯I–C or C–I⋯Cl–PtCl3 moieties, respectively. All XB contacts are clearly observed on the Hirshfeld surfaces; in addition, intermolecular contacts are visualized using noncovalent interactions (NCI) analysis. The steric factor and the number of XB donor centers play important roles in the supramolecular organization of compounds 1·FIB and 2: the bifunctional FIB with two σ-hole donor centers forms an extended structure, including the bridging [PtCl4]2−, whereas the [Ph3PCH2I]+ cation with a σ-hole donor center in a sterically encumbered environment forms only one terminal XB with [PtCl4]2−. The results of DFT calculations followed by topological analysis of the electron density distribution within the framework of the QTAIM method at the ωB97XD/DZP-DKH level of theory reveal that the energies of the attractive intermolecular noncovalent interactions are 6.3–6.4 kcal mol−1 for compound 1·FIB and 2.6–2.9 kcal mol−1 for compound 2.
PPh3]2[PtCl4] with 1,4-diiodotetrafluorobenzene (1·FIB) and the salt [Ph3PCH2I]2[PtCl4] (2), the tetrachloroplatinate(II) anion behaves as a halogen bond (XB)-accepting tecton toward σ-hole-donating organohalogen species to form either C–I⋯Cl–PtCl2–Cl⋯I–C or C–I⋯Cl–PtCl3 moieties, respectively. All XB contacts are clearly observed on the Hirshfeld surfaces; in addition, intermolecular contacts are visualized using noncovalent interactions (NCI) analysis. The steric factor and the number of XB donor centers play important roles in the supramolecular organization of compounds 1·FIB and 2: the bifunctional FIB with two σ-hole donor centers forms an extended structure, including the bridging [PtCl4]2−, whereas the [Ph3PCH2I]+ cation with a σ-hole donor center in a sterically encumbered environment forms only one terminal XB with [PtCl4]2−. The results of DFT calculations followed by topological analysis of the electron density distribution within the framework of the QTAIM method at the ωB97XD/DZP-DKH level of theory reveal that the energies of the attractive intermolecular noncovalent interactions are 6.3–6.4 kcal mol−1 for compound 1·FIB and 2.6–2.9 kcal mol−1 for compound 2.


 Please wait while we load your content...
Please wait while we load your content...