Thermodynamic pathway between the non-polar and ferroelectric polymorphs of guanidinium ethoxysulfonate†
Abstract
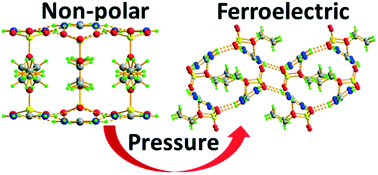
At ambient pressure, guanidinium ethoxysulfonate (GES) crystallizes in two forms, α-GES and β-GES, in which the cations and anions are H-bonded into supramolecular 2D motifs. It has been shown that the non-polar α-GES built of bilayers cannot be transformed into the ferroelectric β-GES of a single-layer architecture, by thermal stimulation. However, this transition can be accomplished under pressure. The transition is irreversible, is of reconstructive-type, and proceeds with a rate depending on numerous factors like the pressure magnitude and duration, and temperature variations. The slow-kinetic processes of transformation, once triggered by pressure, run for a long time after releasing the pressure. The high-pressure dielectric studies revealed the complexity of the p–T phase diagram of β-GES. Three of the four new crystalline phases, which emerge under pressure between the ferroelectric and paraelectric phases, exhibit properties indicating a possible modulation of the crystal structure. The high-temperature phase I of β-GES has been crystallized in situ in a glass capillary and its structure has been determined by single-crystal X-ray diffraction. The structural and calorimetric data evidenced that the transition between phases I and II of β-GES has a reconstructive character, but it is fully reversible.


 Please wait while we load your content...
Please wait while we load your content...