Selective enclathration of xylenols: synergistic effects of mixed hosts†
Abstract
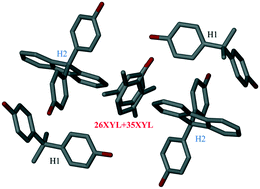
The six xylenol (XYL) isomers can be separated by selective enclathration with the host 4,4-isopropylidene bisphenol, H1. Crystal structures were elucidated for the following single and mixed guest inclusion compounds with H1: H1·34XYL (I), H1·35XYL (II), H1·23XYL·26XYL (III), H1·23XYL·35XYL (IV), where the xylenol isomers are abbreviated as, for example 34XYL for 3,4-xylenol. The crystal structures of selected H1·xylenols showed that there is extensive host⋯host and host⋯guest hydrogen bonding. Competition experiments with equimolar mixtures of pairs of xylenols (XYL) showed that the preference for inclusion was in the sequence 34XYL > 35XYL > 26XYL > 23XYL > 25XYL > 24XYL. By analogy to the Dutch resolution method (in which families of resolving agents are used to achieve chiral separations), two host compounds similar to H1 were used in pairs with H1 to improve the selectivity of the xylenols. 4,4′-(9-Fluorenylidene)bisphenol, H2, and 4,4′(cyclohexylidene)bisphenol, H3, were used in pairs with H1 and were shown to enhance the selectivity of a given xylenol which had been poorly separated by H1 alone. The crystal structure was elucidated for an unusual mixed host–mixed guest inclusion compound, H1·H2·26XYL/35XYL (V).
- This article is part of the themed collections: Host‒Guest chemistry: in honour of Luigi Nassimbeni’s 9th decade and The Cambridge Structural Database - A wealth of knowledge gained from a million structures


 Please wait while we load your content...
Please wait while we load your content...