Lactic-acid enhanced solvothermal crystallization, color-tunable photoluminescence, and thermal stability of h-LaPO4:Ce3+, Tb3+, Sm3+ nanocrystals†
Abstract
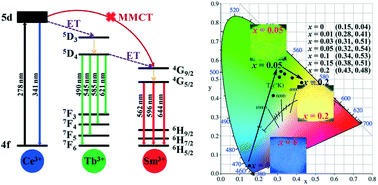
Lactic acid (LA) was originally employed as a cosolvent for solvothermal synthesis of LaPO4:Ce3+, Tb3+, Sm3+ nanocrystal phosphors, whose effect on crystal growth was revealed by the combined techniques XRD, FE-SEM and TEM. It was shown that LA not only remarkably restricted the intrinsic one-dimensional (1D) growth of hexagonal LaPO4 crystals along the [001] direction, resulting in a shape variation from microwires to nanoparticles, but also promoted crystallite growth. With more efficient Ce3+ → Tb3+ energy transfer resulting from larger crystallite size, the h-(La0.90Ce0.05Tb0.05)PO4 nanoparticles presented more intense and thermally stable green emission of Tb3+ as compared to the nanowires. Moreover, a series of (La0.93−xCe0.05TbxSm0.02)PO4 (x = 0–0.40) nanocrystals, with the emission color varying from blue to green and eventually to yellow, were successfully prepared through adjusting the concentration of Tb3+, in which the Tb3+ ion acted as an energy transfer bridge between Ce3+ and Sm3+. The mechanism of Ce3+ → Tb3+ → Sm3+ energy transfer was proposed, and the optimal Tb3+ content for Sm3+ emission was determined to be x = 0.20. The optimized (La0.73Ce0.05Tb0.20Sm0.02)PO4 phosphor exhibited good thermal stability for Sm3+ emission, whose activation energy for thermal quenching was found to be ∼0.33 eV.


 Please wait while we load your content...
Please wait while we load your content...