Two Co-based MOFs assembled from an amine-functionalized pyridinecarboxylate ligand: inorganic acid-directed structural variety and gas adsorption properties†
Abstract
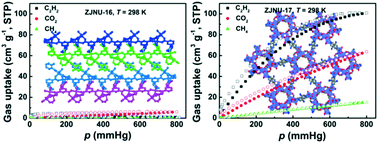
By altering inorganic acid additives under otherwise identical synthetic conditions, two MOFs termed ZJNU-16 and ZJUN-17 were successfully constructed through solvothermal reactions of Co(NO3)2·6H2O and an amine-functionalized pyridinecarboxylate ligand, namely, 5-(3-aminopyridine-4-yl)isophthalic acid. Structural analyses indicated that the two compounds exhibited inorganic acid-guided structural diversities, adopting different SBUs, ligand conformations, and network topologies. ZJNU-16 is a (3,6)-connected network with a discrete trinuclear Co3-based cluster as an inorganic SBU, while ZJNU-17 is a (3,5)-connected framework featuring a nanotubular helical Co–carboxylate chain as an inorganic SBU. Furthermore, their gas adsorption properties towards N2, C2H2, CO2, and CH4 were systematically investigated, revealing the potential of ZJNU-17 for C2H2 separation and purification under ambient conditions. At 298 K and 106.7 kPa, the C2H2 and CO2 uptake capacities reach 100.9 and 63.8 cm3 (STP) g−1, and the IAST-predicted adsorption selectivities of C2H2 over CH4 and CO2 are 15.5 and 2.5, respectively. Although the performance is moderate compared to some reported MOFs, ZJNU-17 is a rare nanotubular helical chain-based MOF exhibiting selective gas separation potential.


 Please wait while we load your content...
Please wait while we load your content...