Phase transformations in a double complex salt of the ruthenium nitrosyl anion and tetraamine-palladium cation†
Abstract
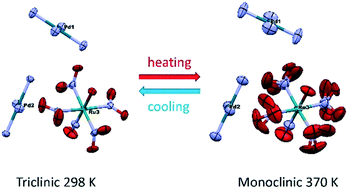
Second-order phase transformation in double complex salt (DCS) [Pd(NH3)4][RuNO(NO2)4OH] was examined by DSC, single-crystal diffraction and powder diffraction. All data confirm the reversible “order–disorder” phase transition without an experimentally detected sharp volume change in the temperature range of 343–363 K. At low temperature, the less symmetric triclinic phase (P![[1 with combining overline]](https://www.rsc.org/images/entities/char_0031_0305.gif) ) is stable, while the monoclinic phase (P2/m) is stable at high temperature. The main difference between crystal structures is the disorder of the ruthenium anion in the monoclinic phase due to the presence of the ruthenium atom in a special position. The additional symmetry plane in the P2/m phase includes ON–Ru–O atoms, resulting in the rotational disorder of NO2 groups. The entropy of transformation determined by differential scanning calorimetry corresponds to order–disorder transformation with a 3-fold increase in microstate number.
) is stable, while the monoclinic phase (P2/m) is stable at high temperature. The main difference between crystal structures is the disorder of the ruthenium anion in the monoclinic phase due to the presence of the ruthenium atom in a special position. The additional symmetry plane in the P2/m phase includes ON–Ru–O atoms, resulting in the rotational disorder of NO2 groups. The entropy of transformation determined by differential scanning calorimetry corresponds to order–disorder transformation with a 3-fold increase in microstate number.


 Please wait while we load your content...
Please wait while we load your content...