Methods for easy recognition of isostructurality – lab jack-like crystal structures of halogenated 2-phenylbenzimidazoles†
Abstract
Tools to describe isostructurality are important in the understanding of close packing principles and in the fine-tuning of crystal properties. In order to present how different methods work in practice, a series of 2-phenylbenzimidazole derivatives substituted on the phenyl ring in the ortho, meta and para positions or simultaneously in two different positions by F, Cl and Br were selected. The flexibility of the phenylbenzimidazole frame permits a gradual isostructural change of the structures with step-by-step alteration of the internal arrangement as well as of the lengths of the unit cells perpendicular to the determining N–H⋯N hydrogen bonded chains. The exchange of the different halogen substituents alters the angle between the neighbouring benzimidazole moieties and the system of the secondary interactions, and finally the isostructurality is terminated. The series of isostructural crystals look like a lab jack lifted at different heights. Although the neighbouring members of the series are highly similar, the extremes of the list vary deliberately keeping the space group and Z. This raises the question about the extents of structural differences what we still consider isostructural. The preference of certain intermolecular interactions divides the investigated isostructural Pbca crystals into two subgroups like a switch. The definition of isostructurality does not consider supramolecular similarity, although it may have a determining role as shown. It is presented how isostructurality can be described by numerical descriptors. Cell similarity (π), isostructurality (Is), and molecular isometricity indices are calculated. Correlations of the molecular conformation, secondary interactions and the crystallographic parameters are revealed by statistical methods. With the use of these methods, we provide an easy way to recognise and to characterize isostructurality. We show that the prerequisites of isostructurality are the similar composition and conformation of the compounds, with their analogous molecular and supramolecular arrangement in the crystals having the same space group and Z. Exploitation of the Cambridge Structural Database for systematical investigations to complete the isostructural series is essential.
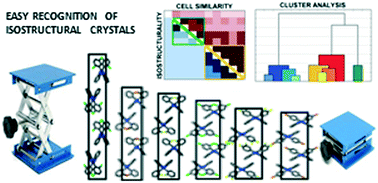
- This article is part of the themed collections: Database Analysis and The Cambridge Structural Database - A wealth of knowledge gained from a million structures


 Please wait while we load your content...
Please wait while we load your content...