Freezing-induced silk I crystallization of silk fibroin†
Abstract
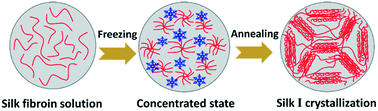
Thermal annealing strongly impacts the crystallization of proteins. Despite the fundamental importance of the crystallization of silk fibroin, the underlying crystallization kinetics of silk fibroin in the freezing state has not been studied in detail. Silk I is a necessary intermediate crystalline structure that was originally found in the natural spinning dope of silkworms. In the present study, water-insoluble silk fibroin materials with the silk I crystalline structure were prepared directly from an aqueous silk fibroin solution by a facile freezing–annealing treatment. Water plasticization significantly reduced the glass-transition temperature of the frozen aqueous silk fibroin solution system to −30–−19 °C. XRD results clearly showed the noncrystalline-to-silk I transition process of frozen silk fibroin during annealing. The results suggested that the mechanism to control silk I crystallization involves freezing-generated concentration and thermodynamically driven assembly of silk fibroin. The biodegradation properties of the freeze-annealed silk fibroin samples were investigated, indicating that the enzyme degradation rate can be regulated over a large scale by controlling the crystallization of silk fibroin. Freezing-induced silk I crystallization offers a simple and entirely green approach for the formation of silk I structure, in turn, providing control of the properties of silk fibroin biomaterials.


 Please wait while we load your content...
Please wait while we load your content...