Nanosheet-assembled carbonated hydroxyapatite microspheres prepared by an EDTA-assisted hydrothermal homogeneous precipitation route
Abstract
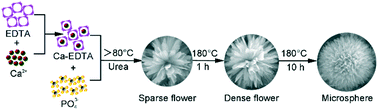
Carbonated hydroxyapatite (CHA) microspheres assembled from nanosheets were synthesized using disodium ethylene diamine tetraacetate (Na2EDTA) as the chelating agent and urea as the pH regulator. By adjusting the hydrothermal treatment temperature and time, the morphologies and three-dimensional architectures of the CHA products were well controlled. The phase, morphology, and particle size distribution of the as-synthesized microspheres were characterized by X-ray diffraction, scanning electron microscopy and laser diffraction particle size analysis. Results show that relatively high reaction temperatures could promote the formation of microspheres. The spherical structures were transformed from sparse to dense with prolonged reaction time. Well-rounded CHA microspheres with a mean diameter of ∼30 μm can be produced at 180 °C for 10 h. The underlying mechanism for the formation of CHA microspheres in the presence of Na2EDTA under hydrothermal homogeneous precipitation conditions was proposed. Flower-like CHA crystals formed, gathered continuously and finally grew into CHA microspheres with increased temperature and prolonged reaction time. This study provides an easy way to obtain well-rounded CHA microspheres with a relatively large size for biomedical applications.


 Please wait while we load your content...
Please wait while we load your content...