Mechanisms of crystallisation in polysorbates and sorbitan esters†
Abstract
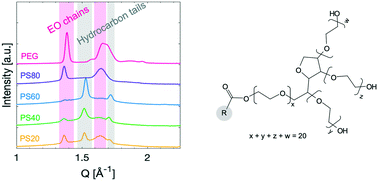
Polysorbates (PS), commonly known as Tween™, are some of the most extensively used excipients and protein stabilisers in biopharmaceutical products worldwide. It is stipulated in the pharmacopoeia specifications that these ethoxylated surfactants are complex mixtures comprised of a wealth of molecular species. While little is known about the propensity of PSs to crystallise, they are used in applications ranging from food products, cosmetics, different types of drug dosage forms like creams and oral products to parenteral applications. However, in recent years a range of issues and safety concerns have appeared when using them for stabilising biopharmaceutical products including precipitation, particle formation, and adverse biological effects. Therefore, the aim of this study was to thoroughly characterise the thermotropic behaviour and mechanism of crystallisation of polysorbates with different hydrocarbon tails and their non-ethoxylated sorbitan ester equivalents for comparison. A systematic and comprehensive product characterisation was carried out, taking advantage of a combination of complementary techniques such as differential scanning calorimetry, matrix assisted laser desorption ionisation time-of-flight and small- and wide-angle X-ray diffraction. We show that polysorbate 80, having an unsaturated hydrocarbon tail, crystallises by the ethylene oxide chains in the headgroup. Polysorbate 20, 40, and 60, containing saturated hydrocarbon esters tails, crystallise not only by the ethylene oxide chains but also by their hydrocarbon tails. An analogous behaviour was observed for the PS non-ethoxylated equivalents, the sorbitan esters. Sorbitan esters with saturated hydrocarbon tails displayed a crystallisation of the tail upon cooling, whereas the sorbitan ester with unsaturated hydrocarbon tail displayed no crystallisation.


 Please wait while we load your content...
Please wait while we load your content...