Substituent effects on the crystallization mechanisms of 7-chloro-4-substituted-quinolines†
Abstract
The crystallization mechanisms of a series of fourteen 7-chloro-4-substituted-quinolines (substituents: (1) OCH3, (2) OCH2CH3, (3) OCH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2, (4) O(CH2)2OH, (5) O(CH2)3OH, (6) NH(CH2)2CH3, (7) NH(CH2)3CH3, (8) NH(CH2)2OH, (9) NHCH(CH2CH3)CH2OH, (10) NH(CH2)2Cl, (11) NHN
CH2, (4) O(CH2)2OH, (5) O(CH2)3OH, (6) NH(CH2)2CH3, (7) NH(CH2)3CH3, (8) NH(CH2)2OH, (9) NHCH(CH2CH3)CH2OH, (10) NH(CH2)2Cl, (11) NHN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(C6H5), (12) NHN
CH(C6H5), (12) NHN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(2-FC6H4), (13) NHN
CH(2-FC6H4), (13) NHN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(3-FC6H4), and (14) NHN
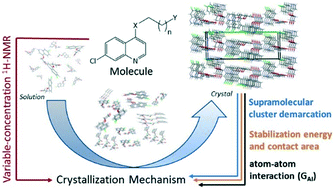
CH(3-FC6H4), and (14) NHN![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(4-FC6H4)) were proposed based on a retrocrystallization approach using the supramolecular cluster as demarcation. Crystallization mechanism stage parameters – NCG% and NG/NC – were determined. The 4-substituents present in quinolines caused six different mechanisms, starting from the monomers in solution to dimers, 1D nuclei, and 2D nuclei (6, 11, 12); 2D nucleus formation (5); 1D nuclei to 2D nuclei (7, 9); concomitant dimers and 1D nuclei to a 3D crystal (8); 1D nuclei directly to a 3D crystal lattice (1–4, 10, 14), and dimers to 2D nuclei and then to a 3D crystal (13). Analysis of GAI showed atom⋯atom intermolecular interactions in the proposed first nuclei for compounds 4 and 5. The nucleation process inferred in the solid state was partially confirmed during the formation of the proto-crystal in solution due to the changes of the hydrogen chemical shift in variable-concentration 1H-NMR experiments. Results revealed that the size of the alkyl chain and the presence of different functional groups in the 4-substituents influenced the crystallization process.
CH(4-FC6H4)) were proposed based on a retrocrystallization approach using the supramolecular cluster as demarcation. Crystallization mechanism stage parameters – NCG% and NG/NC – were determined. The 4-substituents present in quinolines caused six different mechanisms, starting from the monomers in solution to dimers, 1D nuclei, and 2D nuclei (6, 11, 12); 2D nucleus formation (5); 1D nuclei to 2D nuclei (7, 9); concomitant dimers and 1D nuclei to a 3D crystal (8); 1D nuclei directly to a 3D crystal lattice (1–4, 10, 14), and dimers to 2D nuclei and then to a 3D crystal (13). Analysis of GAI showed atom⋯atom intermolecular interactions in the proposed first nuclei for compounds 4 and 5. The nucleation process inferred in the solid state was partially confirmed during the formation of the proto-crystal in solution due to the changes of the hydrogen chemical shift in variable-concentration 1H-NMR experiments. Results revealed that the size of the alkyl chain and the presence of different functional groups in the 4-substituents influenced the crystallization process.


 Please wait while we load your content...
Please wait while we load your content...