Crystal nucleation of salicylamide and a comparison with salicylic acid†
Abstract
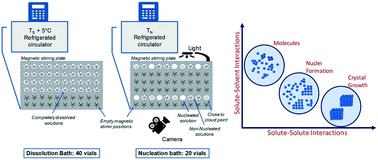
Crystal nucleation of salicylamide has been investigated in four different solvents. The interfacial energy as determined within the classical nucleation theory is found to increase in the order acetonitrile < ethyl acetate < acetone < methanol. However, this is compensated for by a similar increase in the pre-exponential factor by which the overall influence of the solvent on the nucleation becomes weaker. In the evaluation, an approximate correction for solution activity coefficient ratios is used in the representation of the driving force. The nucleation results are compared with the nucleation of a similar compound: salicylic acid. It is found that the influence of the solvent has a similar trend for the two solutes while the nucleation of salicylic acid is somewhat easier. Possible explanations for this are discussed attempting to progress the rationalization of crystal nucleation of organic solutes in solution. It is found that the attachment factor in the different systems as predicted from physical properties correlates well with the experimentally determined pre-exponential factor.


 Please wait while we load your content...
Please wait while we load your content...