Evidence of reaction intermediates in microwave-assisted synthesis of SHG active α-La(IO3)3 nanocrystals†
Abstract
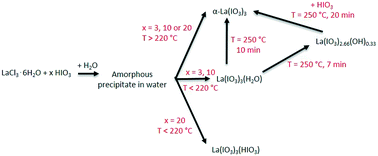
We explore here how, under microwave assisted hydrothermal process, various synthesis conditions influence the formation of different lanthanum iodate compounds: α-La(IO3)3, La(IO3)3(H2O), La(IO3)3(HIO3) and La(IO3)2.66(OH)0.66. Whatever the initial [La3+] : [IO3−] ratio, α-La(IO3)3 crystallizes if the synthesis temperature exceeds 220 °C. Phase transformations in solid state or under hydrothermal synthesis are also discussed. Interestingly, we evidence the full phase transformation under hydrothermal synthesis from the new hydrate form La(IO3)3(H2O) into stable and optically active α-La(IO3)3 through an appropriate hydrothermal process. This evolution can be explained by structural rearrangements of the coordination sphere of lanthanum atoms, stabilizing the structure.


 Please wait while we load your content...
Please wait while we load your content...