Self-assembly of three-dimensional oxalate-bridged alkali(i)–lanthanide(iii) heterometal–organic frameworks†
Abstract
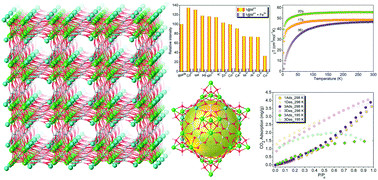
Three isostructural three-dimensional (3D) oxalate bridged alkali(I)–lanthanide(III) heterometal–organic frameworks with the general formula [Ln8Na4(ox)10(μ3-OH)8]·MeOH·10H2O (Ln = Tb (1), Dy (2), Er (3)) have been assembled from the solvothermal reactions of oxalic acid (H2ox) with Ln(NO3)3 and Na2CO3. These compounds crystallize in the tetragonal I4/m space group and exhibit a 3D six-connected uninodal net of pcu alpha-Po primitive cubic type based on tetranuclear [Ln4(μ3-OH)4] cubane clusters. Among them, compound 1 exhibits a multifunctional luminescent probe property for the detection of acetone, toluene, and Fe3+ ions based on fluorescence quenching mechanisms. The magnetic investigations of 1–3 indicate antiferromagnetic coupling between the spin centers Ln3+ ions. In addition, 1 and 3 show negligible adsorption capacity for carbon dioxide.


 Please wait while we load your content...
Please wait while we load your content...