Effect of stepwise protonation of an N-containing ligand on the formation of metal–organic salts and coordination complexes in the solid state†
Abstract
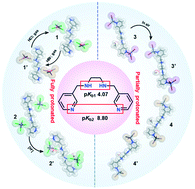
By controlling the protonation level of the ligand (N,N′-bis(pyridin-3-ylmethyl)ethane-1,2-diamine, L), a series of metal–organic salts (MOSs) (1–2′) and coordination complexes (3–4′) were synthesized with metal halides MX2 (M = Cu, Zn; X = Cl, Br, I). The structural diversities of 1–4′ revealed that the solvents, pH value and protonation levels of the ligand played significant roles in affecting the coordination conformations during the structural self-assembly process. The crystals of [4HL]4+·2[CuX4]2− (X = Cl 1, Br 1′) showed unusual reversible transformations by desorption/absorption of gaseous HCl–HBr and the progress of the solid–gas reaction was monitored by ex situ X-ray powder diffraction. Crystal [4HL]4+·2[ZnCl4]2− (2) transformed into [4HL]4+·2[ZnCl4]2−·2H2O (2′) due to the variation in concentration of [H+] in the mother solution. Crystal [Zn2(2HL)I6]·2CH3OH (3) exhibited a phase transition upon exposing the powder to air for 24 hours, giving rise to a new phase [Zn2(2HL)I6]·2H2O (3′).


 Please wait while we load your content...
Please wait while we load your content...