Growth kinetics of curcumin form I†
Abstract
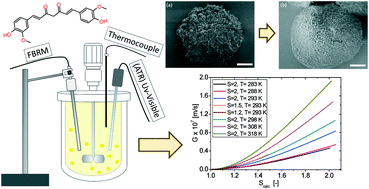
The growth rate of pure curcumin particles (CUR) in supersaturated propan-2-ol solutions has been determined by seeded isothermal desupersaturation experiments at different temperatures (283–318 K). In situ ATR UV-vis and FBRM were used to monitor the solution concentration over time and to verify that no nucleation was occurring. Principal component analysis (PCA) was applied to the UV-vis spectra to determine the supersaturation decay over time. The growth rate is examined by fitting the power law empirical models and the Burton Cabrera Frank (BCF) and birth and spread (B + S) mechanistic models to the experimental desupersaturation data. The growth rate is low, and the growth order (1.75) and the activation energy (38 kJ mol−1) are high, all suggesting that growth is surface integration controlled. Fitting the B + S model, the solid–liquid interfacial energy is estimated to be 2.7 mJ m−2. Approximate surface diffusion rates are extracted and the mean surface diffusion distance is estimated to be 2 nm. The growth rate is clearly lower than that found for other compounds of lower molecular weight. This originates from a higher interfacial surface energy and a slower surface diffusion.


 Please wait while we load your content...
Please wait while we load your content...