Lilac flower-shaped ZnCo2O4 electrocatalyst for efficient methanol oxidation and oxygen reduction reactions in an alkaline medium†
Abstract
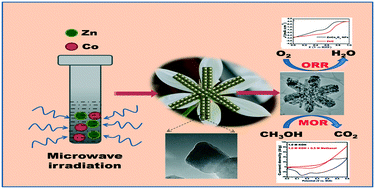
The oxygen reduction reaction (ORR) plays a vital role in electrochemical energy conversion and energy storage devices such as metal–air batteries and alkaline fuel cells. The development of proficient, noble metal-free catalysts for electrocatalytic ORRs is one of the main challenges impeding fuel cell research. ZnCo2O4 lilac flowers (LFs) were synthesized using a rapid microwave irradiation method, at 160 °C for 15 min, followed by annealing, at 400 °C for 4 h. XRD confirms their high crystallinity and phase purity. HR-TEM images show several smaller, primary nanoparticle building blocks, which combine to form lilac flower-like structures, through a self-assembly process. The methanol oxidation reaction (MOR) and ORR were evaluated using ZnCo2O4 LFs as a catalyst. The stability and durability of the ZnCo2O4 LF catalyst were analyzed by chronoamperometry (CA) measurements and CA responses for the ORR on ZnCo2O4 LFs at −0.5 V vs. RHE, in O2-saturated 0.1 M KOH electrolyte. These tests showed that the catalyst exhibited remarkable MOR and ORR activity in alkaline media.


 Please wait while we load your content...
Please wait while we load your content...