A dual-functional metal phosphate for high proton conduction and selective luminescence turn-on sensing of Co2+ ions†
Abstract
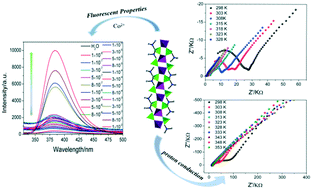
In this work, {[Zn(H2PIPZ)](H2O)}n was synthesized by self-assembly of an N,N′-piperazinebis(methylenephosphonic acid) [H4PIPZ] ligand and zinc chloride under hydrothermal conditions. The Zn2+ ion is four-coordinated by oxygen atoms from four different H2PIPZ2− ligands to form a tetrahedral configuration. The Zn2+ tetrahedral units are further connected by a phosphate group of the H2PIPZ2− ligand to form a 3D structure. AC analysis shows that the proton conductivity of compound 1 is excellent and by introducing 1 into PVA to make composite membranes, the proton conductivity of composite membrane 1@PVA-10 (10% wt) reached 2.78 × 10−4 S cm−1 at 98% RH and 353 K, which is approximately ten times higher than that of 1. In addition, compound 1 has excellent selectivity and sensitivity to cobalt ions in water due to turn on fluorescence. The detection limit is 1.0 × 10−8 M. In contrast, the Fe3+ ion has a turn off fluorescence effect on 1 and the detection limit is 1.0 × 10−6 M.


 Please wait while we load your content...
Please wait while we load your content...