3-(4-Methylthiophenyl)acetylacetone – ups and downs of flexibility in the synthesis of mixed metal–organic frameworks. Ditopic bridging of hard and soft cations and site-specific desolvation†
Abstract
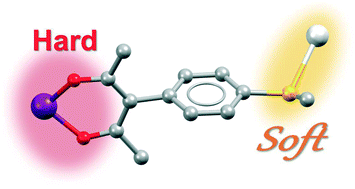
3-(4-Methylthiophenyl)pentane-2,4-dione (HacacSMePh) combines a Pearson-hard β-diketone and a soft thioether group in its periphery; after deprotonation, it may act as a heterobifunctional linker between metal cations. Coordination of the β-diketo site to trivalent cations afforded a series of M(acacSMePh)3 metal complexes with dangling thioether moieties. Crosslinking of the mononuclear M(acacSMePh)3 building units via the SMe functionalities with AgI cations resulted in mixed metal–organic frameworks (MMOFs). All bimetallic networks with MIII = Co, Al, Cr, Fe, Ga are isostructural. Statistical occupancy of the MIII site by two different cations may be possible and has been proven for AlIII and GaIII. The MMOFs are semiporous and contain two co-crystallized chloroform molecules in the asymmetric unit. One of these CHCl3 molecules may be partially removed while maintaining the structural integrity of the network.


 Please wait while we load your content...
Please wait while we load your content...