Control of the morphology, specific surface area and agglomeration of precipitated calcium carbonate crystals through a multiphase carbonation process†
Abstract
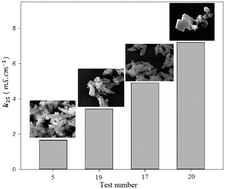
In this study, precipitated calcium carbonate (PCC) has been produced using a semi-continuous carbonation process within a Ca(OH)2(s)–CO2(g)–H2O system inside a bench-scale reactor. In order to understand the effects of the temperature, solid percentage of milk of lime (MOL), CO2 gas injection rate, and agitation rate on the conductivity, specific surface area, microporous surface area (and subsequently agglomeration), and reaction termination time, an experimental procedure was used based on central composite design (CCD). Conductivity and pH were applied as the parameters controlling the reaction termination time. Using the data obtained from multiple regression analysis, the effects of the parameters on the abovementioned responses were obtained. The results showed that the conductivity has a direct relationship with the morphology; therefore, the conductivity level was considered as a parameter to determine the morphology. Moreover, the microporous surface area of the PCC was considered to determine the agglomeration. In this study, ultra-pure PCC samples with high specific surface areas and controlled morphologies (scalenohedral, cluster scalenohedral, rhombohedral, vaterite or chain-like agglomerates) were produced without adding any crystal growth modifier (CGM). The obtained PCC showed dimensions of 20 nm to 2 μm, with specific surface areas of 5.99–26.69 m2 g−1, purity of 99.86%, and a controlled morphology.


 Please wait while we load your content...
Please wait while we load your content...