Changing the shape and optical properties of CsPbBr3 perovskite nanocrystals with hydrohalic acids using a room-temperature synthesis process†
Abstract
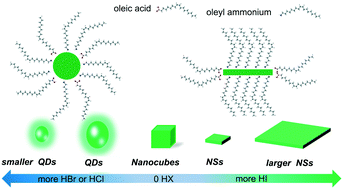
Here, we present that the shape and optical properties of CsPbBr3 nanocrystals (NCs) could be tuned by addition of various hydrohalic acids (HX, X = Cl, Br, I). The addition of hydrochloric acid (HCl) and hydrobromic acid (HBr) turns the nanocrystals from nanocubes (NCUs) to quantum dots (QDs). The optical properties are regulated by the composition and quantum confinement effect, while the hydrobromic acid (HBr) addition results in nanosheets (NSs), without a photoluminescence (PL) peak shift. Based on various characteristics, we speculate that the addition of HX leads to protonation of oleylamine from oleylamine into oleylammonium cations that compete with Cs+ during crystallization. The oleylammonium cations at the growing surface of NCs block further growth in the favored direction. Our study provides a facile strategy for tuning and manipulating the shape, size, and optical properties of all-inorganic CsPbX3 NCs and aids in the further exploration of the growth mechanism of perovskite NCs.


 Please wait while we load your content...
Please wait while we load your content...