Supramolecular organisation of sulphate salt hydrates exemplified with brucine sulphate†
Abstract
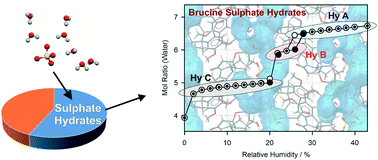
The solid form landscape of brucine sulphate (BS) was elucidated, resulting in three hydrate forms (HyA–C) and amorphous BS. Interconversion of the hydrates of BS with small changes in the relative humidity complicated identifying and characterising the solid forms. The hydrate obtained from crystallisation experiments (from water), HyA, is the only solid form described in the literature. The other two hydrates were produced by dehydration starting from the known hydrate. HyA contains 6.5 to 7.4 molecules of water per BS and is only stable in the relative humidity (RH) range ≥26% at room temperature (RT). HyB is only observable in a very narrow RH window (22–25%) at RT and shows a hexahydrate stoichiometry. At RH values ≤20%, the third hydrate, HyC, forms. Similar to HyA, the latter hydrate shows a variable water content of five or less water molecules per BS. Removal of the essential water molecules stabilising the hydrate structures causes the collapse to the amorphous state, a process which was not completed within 3.5 years of storing HyC under driest conditions (approx. 0%) at room temperature. Only the combination of intermolecular interaction and electronic structure calculations with thermal analytical techniques, X-ray diffraction, IR spectroscopy and gravimetric moisture (de)sorption studies and careful control of the external conditions allowed the discovery and rationalisation of the three hydrates of BS. The investigations on BS were complemented with an exploitation of the Cambridge Structural Database (CSD) to unravel the incidence of hydrates of sulphate salts. The analysis indicates that 56.5% of the sulphate salts (C, H, N, O, and S atoms only) are hydrate structures, with higher hydrates being more commonly present amongst sulphate salts than amongst all organic hydrates.
- This article is part of the themed collection: The Cambridge Structural Database - A wealth of knowledge gained from a million structures


 Please wait while we load your content...
Please wait while we load your content...