Temperature-controlled hydrothermal synthesis of γ-MnOOH, Mn3O4 and MnCO3 on a carbon cloth for supercapacitor applications†
Abstract
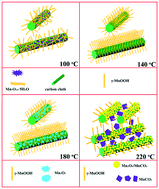
In this study, a reaction temperature-controlled synthesis of γ-MnOOH, Mn3O4 and MnCO3 (and also their mixture) on carbon cloth via a hydrothermal method is investigated, and the formation mechanism is discussed. The experiments demonstrate that the reducing ability of the carbon cloth tuned by the reaction temperature dominates the compositions and morphologies of the final products. A clear conversion process from Mn7O13·5H2O to MnCO3via γ-MnOOH and Mn3O4 on carbon cloth with an increase in the reaction temperature from 100 °C to 220 °C is confirmed. The electrochemical performance of γ-MnOOH on carbon cloth synthesized at 140 °C and MnCO3 as the main component on carbon cloth synthesized at 220 °C are investigated and both show high performance.


 Please wait while we load your content...
Please wait while we load your content...