Solution chemistry and phase solubility diagrams of CL-20/MTNP energetic cocrystals†
Abstract
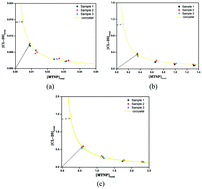
The solubility behavior and solution chemistry of CL-20/MTNP (2,4,6,8,10,12-hexanitro-hexaaza-isowurtzitane/1-methyl-3,4,5-trinitropyrazole) energetic cocrystals in organic solvents were first investigated to offer some important information on thermodynamics. The phase solubility diagram (PSD) and eutectic point (Keu1) of three solvents were also determined. Moreover, the solubility behavior of CL-20/MTNP energetic cocrystals was explained using the solubility product and solution complexation theories. The solution complexation results indicated that the CL-20/MTNP cocrystal has the strongest complexation effect in ethanol. The Keu1 values were all below 1 in three studied solvents, suggesting that the CL-20/MTNP cocrystal was less soluble than the other two pure components. Furthermore, studying the solubility behavior and solution chemistry of the CL-20/MTNP cocrystals has provided further insights into the screening, preparation and scaling-up of energetic cocrystals.


 Please wait while we load your content...
Please wait while we load your content...