From an organic ligand to a metal–organic coordination polymer, and to a metal–organic coordination polymer–cocrystal composite: a continuous promotion of the proton conductivity of crystalline materials†
Abstract
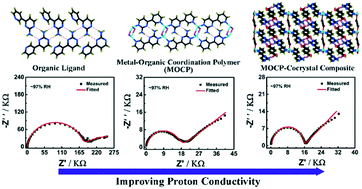
Improving proton conductivity is of great importance for the further application of metal–organic frameworks (MOFs) or metal–organic coordination polymers (MOCPs) in proton exchange membrane fuel cells. In this paper, we proposed a new approach to increase proton conductivities in MOCPs coming from organic ligands, e.g. coordination inducement and MOCP–cocrystal composite formation strategies, which is based on the potential function of organic ligands. By employing the approach, we have successfully synthesized and characterized three crystalline compounds, PPA (1), [Cu(PPA)I] (2) and [Co(PPA)2(BDC)(H2O)2·(PPA)2(H2BDC)2(H2O)] (3) (PPA = 4-(3-pyridinyl)-2-amino pyrimidine, H2BDC = 1,4-benzenedicarboxylic acid). These compounds show high water stabilities and different proton conducting behaviours, especially compound 3 with a proton conductivity of 2.29 × 10−4 S cm−1 at 325 K and ∼97% RH, which is higher than or comparable to those of some reported compounds. More importantly, a persistent increase in proton conductivity is observed from 1 to 3, which is about one order of magnitude. Furthermore, single crystal analyses show that coordination and hydrogen-bonding interactions play a crucial role in improving proton-conductive properties for these compounds. The study reveals that it is practical to boost the proton conductivity of crystal materials by means of their intrinsic merits and the strategy of molecular design.


 Please wait while we load your content...
Please wait while we load your content...