An investigation of the polymorphism of a potent nonsteroidal anti-inflammatory drug flunixin†
Abstract
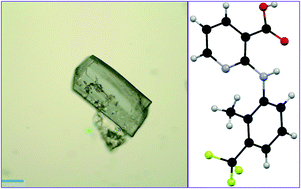
Flunixin [2-(3-trifluoromethyl-2-methyl-phenylamino)-nicotinic acid, FLX], a potent nonsteroidal anti-inflammatory drug widely used in veterinary medicine, was found to exist in at least two crystal forms (I and II), in contrast to clonixin [2-(3-chloro-2-methyl-phenylamino)-nicotinic acid, CLX], which exists in four solvent-free forms and multiple solvates. Form I was harvested from a variety of solvents and characterized by single-crystal X-ray diffraction, PXRD, FT-IR, and Raman spectroscopy. Its crystal structure is sustained on the strong acid–pyridine hydrogen bond. Form II was generated by thermal treatment of form I. Other aspects of this polymorphic system were investigated both experimentally and theoretically. Quantum chemistry calculations were performed to shed light on the lack of polymorphism from solution-phase crystallization. Conformational scan of the dihedral angle C2–N7–C8–C9 (τ) revealed two stable conformations, one with τ near 170°, and the other near 70°, corresponding to the molecule in the crystal. Hirshfeld analysis accounted for the major intermolecular interactions contributing to the overall stability of the crystal.


 Please wait while we load your content...
Please wait while we load your content...