A soft chemistry approach to preparing (de)sodiated transition-metal hydroxy molybdates
Abstract
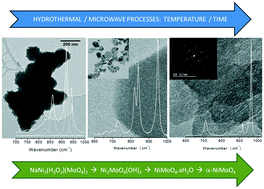
A soft chemistry approach was applied to synthesize de(sodiated) transition-metal hydroxy molybdates under hydrothermal conditions. Different NaM2(H3O2)(MoO4)2 and M2MoO4(OH)2 compositions (M = Ni, Zn) were prepared at mild temperatures (110–250 °C) for times ranging from 10 min to 24 h. Sodiated compounds were obtained after a microwave-assisted hydrothermal process at 150 °C (10–120 min) for zinc-based materials. Besides, conventional heating destabilized the sodiated NaZn2(H3O2)(MoO4)2 phase at 110 °C, thus increasing the amount of Zn2MoO4(OH)2 at temperatures up to 250 °C. NaNi2(H3O2)(MoO4)2 phases were noted only for 10 min under microwave irradiation, while longer times promoted a chemical reaction towards a new desodiated phase. This novel Ni2MoO4(OH)2 phase was also obtained by conventional hydrothermal processing (110–250 °C, 24 h). Slow precipitation rates yielded a hydrated nickel molybdate (triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) after coprecipitation followed or not by microwave irradiation. Raman spectroscopy analysis results showed a good agreement with experimental results and group-theory calculations for both sodiated and desodiated materials. A temperature-induced polymorphic transformation was observed from NiMoO4·xH2O (triclinic, P
) after coprecipitation followed or not by microwave irradiation. Raman spectroscopy analysis results showed a good agreement with experimental results and group-theory calculations for both sodiated and desodiated materials. A temperature-induced polymorphic transformation was observed from NiMoO4·xH2O (triclinic, P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) to α-NiMoO4 (monoclinic, C2/m). For all the obtained polymorphs, the relevant Raman modes were depicted, allowing us to determine their spectroscopic fingerprints. The results constitute an important basis on the understanding of the synthesis of (de)sodiated transition-metal hydroxy molybdates under hydrothermal conditions.
) to α-NiMoO4 (monoclinic, C2/m). For all the obtained polymorphs, the relevant Raman modes were depicted, allowing us to determine their spectroscopic fingerprints. The results constitute an important basis on the understanding of the synthesis of (de)sodiated transition-metal hydroxy molybdates under hydrothermal conditions.


 Please wait while we load your content...
Please wait while we load your content...