Solution-synthesis of Sb2Se3 nanorods using KSeCN as a molecular selenium source†
Abstract
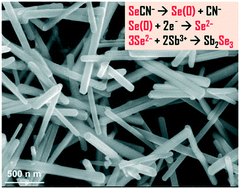
Antimony selenide (Sb2Se3) is a low-toxic, element-abundant, narrow bandgap (Eg = ∼1.1–1.3 eV) semiconductor that shows potential for UV-Visible-Near-infrared optoelectronic applications. This paper reports the use of potassium selenocyanate (KSeCN) as a novel molecular selenium source to synthesize Sb2Se3 uniform nanorods. The resulting nanorods have been carefully characterized and are found to exhibit decent photoconductivity as well as broad-spectrum optical absorption with an Eg value of ∼1.35 eV. A molecular reaction mechanism is rationally proposed and evidenced for forming Sb2Se3, which is related to the thermal decomposition of selenocyanate (SeCN−) anions through the cleaving of Se–CN bonds to elemental Se(0), followed by its reduction to Se2− anions. Our work using KSeCN offers an alternative method for the synthesis of metal selenides with desirable nanostructures and properties.


 Please wait while we load your content...
Please wait while we load your content...