Correlation between microstructure, cation distribution and magnetism in Ni1−xZnxFe2O4 nanocrystallites†
Abstract
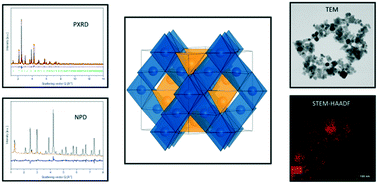
Hydrothermal synthesis of magnetic ferrite spinel nanoparticles, with composition Ni1−xZnxFe2O4 resulted in sizes below 27 nm in the entire composition range (x = 0.0–1.0). The nanoparticles were analyzed with regard to their microstructure, crystal structure and magnetic properties. Combined Rietveld refinements of powder X-ray diffraction (PXRD) and neutron powder diffraction data (NPD), were used to test and compare different models for the cation distribution between the tetrahedral and octahedral sites in the spinel structure. The structural modeling reveals that the Ni2+ ions have a strong preference for the octahedral coordination irrespective of composition, which is consistent with the inverse spinel structure of bulk NiFe2O4. However, the Zn2+ ions are found to partly occupy octahedral sites, despite their preference for tetrahedral coordination in the bulk. Annealing of the nanoparticles in air for 4 h at 600 °C causes a transition towards a more bulk-like cation configuration. The powder diffraction data were complemented by transmission electron microscopy (TEM), scanning transmission electron microscopy with a high-angle annular dark field detector (STEM-HAADF), energy-dispersive X-ray spectroscopy (EDS) and vibrating sample magnetometry (VSM). Quantitative analysis of EDS spectra shows that the desired stoichiometries were obtained. The highest saturation magnetization, of 66 Am2 kg−1, is achieved for Ni0.6Zn0.4Fe2O4. Furthermore, the saturation magnetization obtained from VSM was compared to the results from atomic magnetic refinements based on the neutron powder diffraction data. The present study confirms that the cation distributions observed in nanocrystalline ZnFe2O4 and NiFe2O4 are maintained in the mixed spinel Ni1−xZnxFe2O4.


 Please wait while we load your content...
Please wait while we load your content...