Tuneable reduction of cymantrenylboranes to diborenes or borylene-derived boratafulvenes†
Abstract
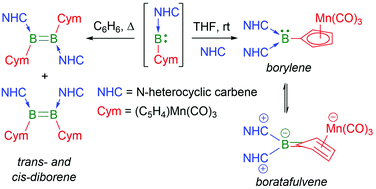
Whereas the reduction of N-heterocyclic carbene (NHC)-stabilised cymantrenyldibromoboranes, (NHC)BBr2Cym, in benzene results in the formation of the corresponding diborenes (NHC)2B2Cym2, a change of solvent to THF yields a borylene analogue of the form (NHC)2BCym, stabilised through a boratafulvene/borafulvenium conformation.


 Please wait while we load your content...
Please wait while we load your content...