Molecular layer deposition of Li-ion conducting “Lithicone” solid electrolytes†
Abstract
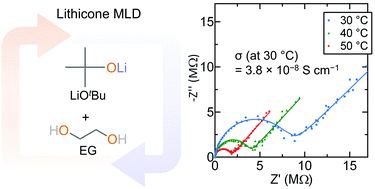
We demonstrate the fabrication of Li-containing (“lithicone”) thin films prepared via molecular layer deposition (MLD) using lithium tert-butoxide and ethylene glycol. X-ray photoelectron spectroscopy reveals that the stoichiometry of the lithicone is Li1.5C2O1.8 (H omitted), with C–O–Li moieties present in the film. The bonding environment of lithicone is distinct from that of lithium carbonate or MLD alucone films. Electrochemical impedance spectroscopy measurements show that annealed lithicone films exhibit room temperature ionic conductivity of 3.6–5 × 10−8 S cm−1 with an activation energy of ∼0.6 eV. The lithicone MLD process provides a pathway to further develop hybrid inorganic–organic Li-ion conducting materials for future battery applications.
- This article is part of the themed collection: 2020 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...