Pyridine mediated transition-metal-free direct alkylation of anilines using alcohols via borrowing hydrogen conditions†
Abstract
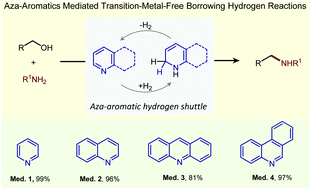
Herein, we report pyridine and other similar azaaromatics as efficient biomimetic hydrogen shuttles for a transition-metal-free direct N-alkylation of aryl and heteroaryl amines using a variety of benzylic and straight chain alcohols. Mechanistic studies including deuterium labeling and the isolation of dihydro-intermediates of the benzannulated pyridine confirmed the role of pyridine and a borrowing hydrogen process operating in these reactions. In addition, we have extended this methodology for the development of dehydrogenative synthesis of quinolines and indoles, as well as the transfer hydrogenation of ketones.


 Please wait while we load your content...
Please wait while we load your content...