Boronic acid-mediated ring-opening and Ni-catalyzed arylation of 1-arylcyclopropyl tosylates†
Abstract
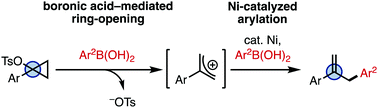
Herein, we describe a protocol for the ring-opening arylation of 1-arylcyclopropyl tosylates, in which boronic acids promote ring-opening and a Ni catalyst facilitates arylation in high regioselectivity. A number of 2-arylated allyl derivatives are synthesized, which are relevant motifs found in biologically active molecules.
- This article is part of the themed collection: 2020 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...