Abstract
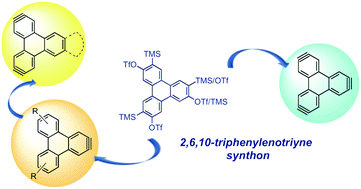
During the last two decades aryne and bisaryne equivalents have been increasingly used as privileged building blocks for the synthesis of polycyclic aromatic hydrocarbons (PAHs). Here we report the synthesis and reactivity of an efficient precursor of the 2,6,10-triphenylenotriyne synthon, which constitutes the best example to date of a trisaryne equivalent on a benzofused polyaromatic core.


 Please wait while we load your content...
Please wait while we load your content...