Complexation behaviour of LiCl and LiPF6 – model studies in the solid-state and in solution using a bidentate picolyl-based ligand†
Abstract
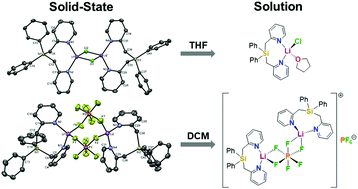
Structural knowledge on ubiquitous lithium salts in solution and in the crystalline state is of paramount importance for our understanding of many chemical reactions and of the electrolyte behaviour in lithium ion batteries. A bulky bidentate Si-based ligand (6) was used to create simplified model systems suitable for correlating structures of LiCl and LiPF6 complexes in the solid-state and in solution by combining various experimental, spectroscopic, and computational methods. Solution studies were performed using 1H DOSY, multinuclear variable temperature NMR spectroscopy, and quantum chemical calculations. [Ph2Si(2-CH2Py)2·LiCl]2 (3) dissociates into a monomeric species (9) in THF. For [Ph2Si(2-CH2Py)2·LiPF6]2 (11), low temperature NMR studies revealed an unprecedented chiral coordination mode (12) in non-coordinating solvents.
- This article is part of the themed collection: 2020 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...