Reactivity prediction in aza-Michael additions without transition state calculations: the Ames test for mutagenicity†
Abstract
Animal testing remains a contentious ethical issue in predictive toxicology. Thus, a fast, versatile, low-cost quantum chemical model is presented for predicting the risk of Ames mutagenicity in a series of 1,4 Michael acceptor type compounds. This framework eliminates the need for transition state calculations, and uses an intermediate structure to probe the reactivity of aza-Michael acceptors. This model can be used in a variety of settings e.g., the design of targeted covalent inhibitors and polyketide biosyntheses.
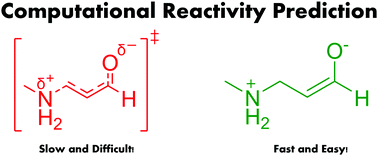


 Please wait while we load your content...
Please wait while we load your content...