Enantioselective dicarbofunctionalization of (E)-alkenyloxindoles with pyridinium salts by chiral Lewis acid/photo relay catalysis†
Abstract
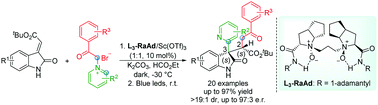
A highly efficient enantioselective dicarbofunctionalization reaction of (E)-alkenyloxindoles with pyridinium salts was realized. The process includes the chiral N,N′-dioxide–Sc(III) complex-catalyzed regio-, diastereo-, and enantioselective [3+2] cycloaddition reaction and the following photo-promoted aza-Norrish II type rearrangement. A series of 2-pyridyl substituted oxindole derivatives were obtained in good yields with moderate to good diastereo- and enantioselectivities.


 Please wait while we load your content...
Please wait while we load your content...