Secondary phosphine oxide-triggered selective oxygenation of a benzyl ligand on palladium†
Abstract
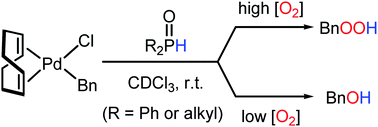
The oxygenation of a benzyl ligand in [PdBnCl(cod)] was dramatically accelerated by using secondary phosphine oxides (SPOs), selectively affording either BnOOH or BnOH, depending on the concentration of O2. The SPOs coordinate to palladium in the form of phosphinous acids, operating as Brønsted acids to facilitate further reaction with O2.


 Please wait while we load your content...
Please wait while we load your content...