Kinetic and mechanistic analysis of a synthetic reversible CO2/HCO2− electrocatalyst†
Abstract
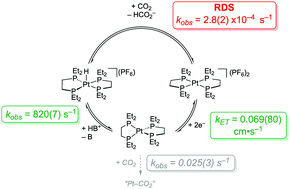
[Pt(depe)2](PF6)2 electrocatalyzes the reversible conversion between CO2 and HCO2− with high selectivity and low overpotential but low rates. A comprehensive kinetic analysis indicates the rate determining step for CO2 reduction is the reactivity of a Pt hydride intermediate to produce HCO2−. To accelerate catalysis, the use of cationic and hydrogen-bond donor additives are explored.
- This article is part of the themed collections: Biohybrid approaches for energy conversion and Bioinspired metal complexes for chemical transformations and catalysis


 Please wait while we load your content...
Please wait while we load your content...
