Recent advances in oxidative allylic C–H functionalization via group IX-metal catalysis
Abstract
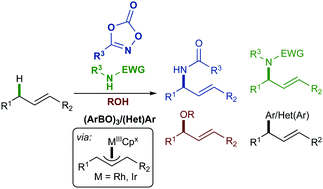
Allylic substitution, pioneered by the work of Tsuji and Trost, has been an invaluable tool in the synthesis of complex molecules for decades. An attractive alternative to allylic substitution is the direct functionalization of allylic C–H bonds of unactivated alkenes, thereby avoiding the need for prefunctionalization. Significant early advances in allylic C–H functionalization were made using palladium catalysis. However, Pd-catalyzed reactions are generally limited to the functionalization of terminal olefins with stabilized nucleophiles. Insights from Li, Cossy, and Tanaka demonstrated the utility of RhCpx catalysts for allylic functionalization. Since these initial reports, a number of key intermolecular Co-, Rh-, and Ir-catalyzed allylic C–H functionalization reactions have been reported, offering significant complementarity to the Pd-catalyzed reactions. Herein, we report a summary of recent advances in intermolecular allylic C–H functionalization via group IX-metal π-allyl complexes. Mechanism-driven development of new catalysts is highlighted, and the potential for future developments is discussed.


 Please wait while we load your content...
Please wait while we load your content...