Three-component ruthenium-catalyzed remote C–H functionalization of 8-aminoquinoline amides†
Abstract
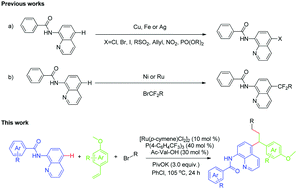
Multicomponent reactions can efficiently construct complex molecular structures from simple precursors. Herein, a novel ruthenium-catalyzed three-component highly selective remote C–H functionalization of 8-aminoquinoline amides has been described. The reaction tolerates a wide range of functional groups, producing arylation/difluoroalkylation products of olefins with potential biological activity and pharmaceutical value. Radical scavenging and radical clock experiments show that a free radical process is involved and a H/D exchange experiment suggests that the reaction might involve ortho-C–H activation of the aromatic ring. A possible mechanism is proposed.
- This article is part of the themed collection: Chemical Communications HOT Articles


 Please wait while we load your content...
Please wait while we load your content...