Organocatalytic asymmetric tandem α-functionalization/1,3-proton shift reaction of benzylidene succinimides with β-trifluoromethyl enones†
Abstract
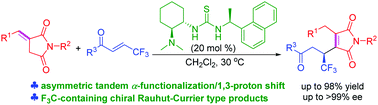
A bifunctional thiourea-catalyzed asymmetric tandem α-functionalization/1,3-proton shift reaction of benzylidene succinimides with β-trifluoromethyl enones has been developed. A series of F3C-containing chiral Rauhut–Currier type products were obtained in moderate to high yields (up to 98%) with excellent enantioselectivities (up to >99% ee). This represents the first example of benzylidene succinimides undergoing tandem α-functionalization/1,3-proton shift in catalytic enantioselective transformation.


 Please wait while we load your content...
Please wait while we load your content...